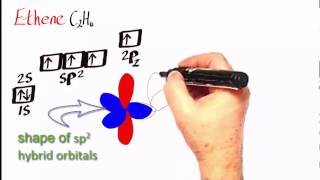
The Calcium Carbonate Double Cycle
We start with limestone, heating it strongly for 1 hour. The quicklime formed is reacted (slaked) with cold water to form slaked lime (LIME). This is calcium hydroxide. A suspension of this in water is filtered...
We start with limestone, heating it strongly for 1 hour. The quicklime formed is reacted (slaked) with cold water to form slaked lime (LIME). This is calcium hydroxide. A suspension of this in water is filtered giving a very dilute solution of the hydroxide as the filtrate. This is limewater. Carbon dioxide is bubbled through the limewater until it goes cloudy and then clear again. Insoluble calcium carbonate and then soluble calcium hydrogen carbonate are formed in turn. The hydrogen carbonate solution is warmed and goes cloudy, due to thermal decomposition back into calcium carbonate.
More...
Collapse
103 Views
Comments (0)
Please log in to post comments.