Lewis Acids and Bases

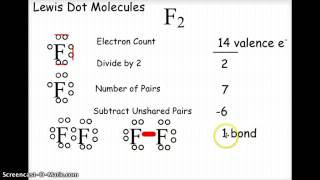
The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ an electron lone pair from another molecule in completing the...
The term Lewis acid refers to a definition of acid published by Gilbert N. Lewis in 1923, specifically: An acid substance is one which can employ an electron lone pair from another molecule in completing the stable group of one of its own atoms. Thus, H+ is a Lewis acid, since it can accept a lone pair, completing its stable form, which requires two electrons.
More...
Collapse
271 Views
Comments (0)
Please log in to post comments.