Ions - Electronegativity and Electron Affinity

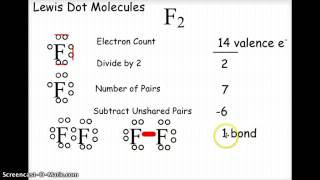
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving the atom a net positive or negative electrical charge.
he electron affinity...
An ion is an atom or molecule in which the total number of electrons is not equal to the total number of protons, giving the atom a net positive or negative electrical charge.
he electron affinity of an atom or molecule is defined as the amount of energy released when an electron is added to a neutral atom or molecule to form a negative ion.
Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom or a functional group to attract electrons (or electron density) towards itself.
More...
Collapse
258 Views
Comments (0)
Please log in to post comments.