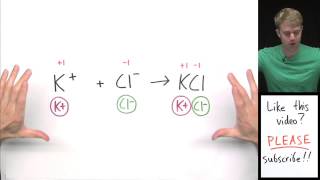Common Ion Effect and Leftward Shift Solubility
The common ion effect is responsible for the reduction in solubility of an ionic precipitate when a soluble compound combining one of the ions of the precipitate is added to the solution in equilibrium with the...
The common ion effect is responsible for the reduction in solubility of an ionic precipitate when a soluble compound combining one of the ions of the precipitate is added to the solution in equilibrium with the precipitate.
More...
Collapse
291 Views
Comments (0)
Please log in to post comments.